Abstract
Introduction:
The observable behavior of a cell, or its phenotype, results from a combination of both its genotype and the microenvironment. Anemia is the defining feature in most patients with myelodysplastic syndromes (MDS), yet very few studies have comprehensively characterized disordered terminal erythroid differentiation (TED) in fresh bone marrow (BM) cells in MDS. One reason could be the lack of appropriate technology for study of TED but another is undue focus on defining gene defects alone at the expense of examining overall cellular function and defects. The current study represents an attempt to develop a phenotypic understanding while simultaneously examining the genotype. TED consists of five distinct phases - proerythroblasts (pros), early basophilic erythroblasts (EBs), late basophilic erythroblasts (LBs), polychromatic erythroblasts (polys), to orthochromatic erythroblasts (orthos) which upon enucleation generate reticulocytes. By examining the dynamic changes in the expression of three salient marker proteins, we quantified erythroblasts at distinct stages of their terminal differentiation in 249 freshly obtained bone marrows (BM) and its association with clinical phenotype including overall survival.
Methods:
Sixteen normal and 233 BMs from MDS (n=215) and secondary acute myeloid leukemia (sAML) patients (n=18) were studied for TED by monitoring the surface expression of glycophorin A, band 3 and integrin alpha-4. Genomic DNA was extracted from BMMNCs using Qiagen's DNAeasy Blood and Tissue kit. In total, 54 genes that are part of the myeloid panel at Cancer Genetics Inc. were sequenced for mutations. Mutations were confirmed using Sanger sequencing for a subset of genes and patients.
Results:
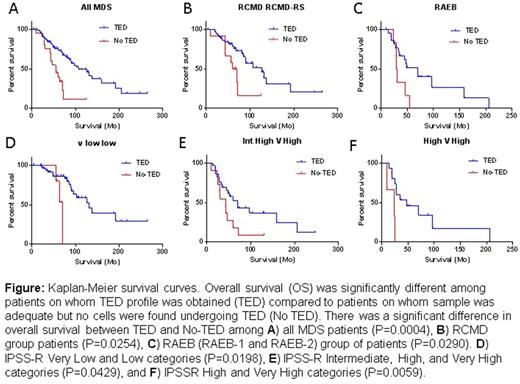
Of 233 BMs, 157 exhibited quantifiable TED profile but did not follow the expected 1:2:4:8:16 doubling pattern showing significantly fewer cells in pro (P=<0.0001) and EB (P=<0.0005). At LB stage, an equal number of cells appeared in normal and MDS samples, but in the poly stage, a significantly higher percentage of cells were detected (P=<0.0001) in MDS with a sudden drop-off in ortho. This suggests a loss of cells somewhere between poly and ortho stages due to cell cycle and/or maturation arrest and resultant apoptosis. A striking reduction in polys was seen as ring sideroblasts (RS) increased, while % of ortho stage cells increased with increasing RS implying lower cell arrest at poly stage with resulting near normal TED progression in MDS with RS. Strikingly, in 33% MDS samples (76/233), there was no quantifiable TED. This group emerged as a distinct clinical entity with more profound anemia (p=0.0003), neutropenia (p=0.0245), higher blast count (p=0.003), significantly worse overall survival (56 versus 103 months, p=<0.0001) and a predominance of cases with mutations in SRSF2 (21/76, p=0.0008). The difference persisted within each subgroup examined; RCMD (p=0.024), RAEB I/II (p=0.029). When TED-positive samples were divided by those with mutations in SF3B1 or SRSF2, overall survival (OS) was worse for those with No-TED and SRSF2+ (p=0.0507). This difference in survival was seen across all IPSS-R categories (Figure 1). In a multivariate Cox regression model, absence of quantifiable TED remained independently significant across IPSS-R categories.
Conclusions:
Terminal erythroid differentiation is profoundly abnormal in all MDS subtypes. Absence of quantifiable TED is more commonly associated with presence of SRSF2 mutations and emerged as a powerful independent prognostic marker of poor overall survival across all IPSS-R categories in MDS. Since MDS is a heterogeneous disease where even the best of prognostic classifications do not fully define the risk of death in a substantial number of cases, we suggest that quantification of TED can serve as an independent prognostic variable. In addition, the findings open a novel area of research which could lead to identification of new therapeutic targets.
Ali: Onconova Therapeutics: Consultancy; Kura Oncology: Consultancy. Hoehn: Janssen: Employment; Johnson & Johnson, LLC: Equity Ownership. Coutinho: Kura Oncology: Consultancy. Lane: Collplant: Consultancy; Kuros: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bone Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Osteology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Graftys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding. Jurcic: Kura Oncology: Research Funding; Incyte: Consultancy; Genentech: Research Funding; Forma Therapeutics: Research Funding; Celgene: Research Funding; Merck: Consultancy; Alexion Pharmaceuticals: Consultancy; Actinium Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Research Funding; Astellas Pharma, Inc: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Research Funding; Syros Pharmaceuticals: Research Funding; Amgen: Consultancy. Raza: Genoptix: Speakers Bureau; Onconova Therapeutics: Research Funding, Speakers Bureau; Syros Pharmaceuticals: Research Funding; Celgene Inc.: Research Funding; Novartis: Speakers Bureau; Kura Oncology: Research Funding; Janssen R&D: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal